

Currently, the Aquatic Vertebrate Platform, supported by Junta de Andalucía, aims to facilitate investigation in aquatic vertebrate models to both national and international researchers and companies. To this end, the scientific manager of the facility, Ana Fernández-Miñán, offers advice on genomic experimentation with the aquatic vertebrates: zebrafish, medaka and Xenopus.
Specifically, services (please click next to download the list of AVP_SERVICES.pdf), collaborations and advice are offered on the following techniques *:
To build expression vectors à la carte we use:
In addition to keep a detailed record of the generated zebrafish transgenic lines, the Platform will also be in charge of their maintenance and distribution to interested researchers. Transgenic lines are available at CABD's Enhancer Screen.
For the experimental testing of your transgenic lines, the following techniques are available:
Morpholinos design and order information can be found at GENE TOOLS.
In collaboration with the Microscopy Facility at CABD (see rates here).
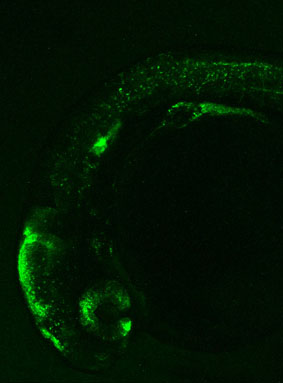
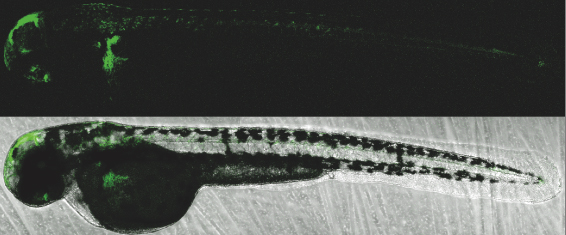
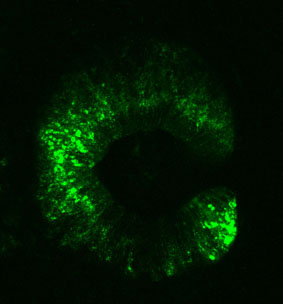
We also offer a range of options for genomic analysis:
An efficient vector designed to test insulator’s functionality in zebrafish F0 injected embryos is used (4).
Insulator’s activity is shown by the ability of the element to downregulate the strong Z48 brain enhancer from the cardiac actin promoter.
Use of the 3C-qPCR technique to confirm distant interaction between two genomic positions, i.e. an enhancer and a promoter
4C-seq technology combines 3C with next generation sequencing. It allows to map the physical interactions' landscape of a given site of interest in a genome-wide manner.
Chromatin inmunoprecipitation using specific antibodies + massive sequencing
MACS (v.1.3.3) algorithm (Peak calling tool)
PinkThingThe Aquatic Vertebrate Platform also offers a Guest Laboratory where to develop collaboration projects under the technical advice from the scientific manager of the facility.
* A, B1 and B2 services are free of charge for Consolider members. Rates for the rest of the services or rates for non-Consolider members must be discussed individually.
Should you be interested in any of the Platform services or in a collaboration, please contact the scientific manager.